Abstract
Background: Advanced stage Mycosis Fungoides (MF) and Sézary Syndrome (SS) are cutaneous T-cell lymphomas (CTCL) associated to dismal prognosis, with 5-year survival rates ranging from 47% for stage IIB to 18% for stage IVA2-IVB and with a high tendency to relapse. Allogeneic hematopoietic stem cell transplantation (allo-HCT) is considered the only curative treatment option. Recently, allo-HCT from haploidentical donor (Haplo-HCT) has been increasingly used also in MF/SS patients. We hereby report an update on long-term outcome of MF/SS patients who underwent allo-HCT at our Center from 2001 to 2022, currently including a larger population with an increased proportion of Haplo-HCT.
Methods: We retrospectively collected clinical data of all consecutive patients with MF/SS transplanted at our Center between September 2001 and May 2022. Conditioning was based on reduced intensity (RIC) regimens, including flaudarabine/cyclophosphamide/TBI200, pentostatin/TBI200, and fludarabine/melphalan in transplants from HLA-identical sibling and unrelated donor (UD), and the thiotepa/fludarabine/cyclophosphamide/TBI200 regimen in Haplo setting. GvHD prophylaxis consisted of CsA, MMF and ATG. Post-transplant cyclophosphamide was added in Haplo-HCT.
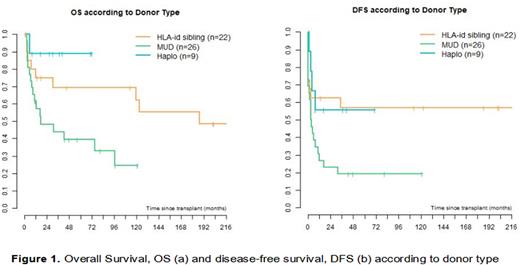
Results: As of August 2022, 57 CTCL patients underwent allo-HCT at our Institution: 22 (38.5%) from a HLA-identical sibling, 13 (23%) from a 10/10 matched UD, 12 (21%) from a 9/10 or 8/10 matched UD, 1 (1.8%) from cord blood, and 9 (15.7%) from a Haplo donor. Median age at transplant was 55 years (range 20-67). Indication for allo-HCT was MF in 36 patients (63%) and SS in 21 patients (37%). Median number of previous treatment lines was 6 (range 2-11) with a median time from diagnosis to transplantation of 46 months (10-274). Disease status at transplant was complete remission (CR) in 14 cases (24.5%), partial remission (PR) in 35 (61.5%) and progressive disease (PD) in 8 (14%). Stem cell source was peripheral blood in 53 patients (92.9%), bone marrow in 3 (5.2%) and cord blood in 1 (1.8%). After a median follow up of 81 months, 30 patients (52%) were alive, of whom 23 (76%) in CR. Acute GvHD occurred in 29 patients (51%; grade III-IV in 13, 23%), while chronic GvHD in 19 (33%; moderate/severe in 10 patients, 17%). Cause of death was disease progression in 17 patients (62.5%), acute GvHD in 4 (15%), infection in 2 (7.5%) and other in 4 (15%). In the whole cohort, 5-yr overall survival (OS) and disease-free survival (DFS) were respectively 57.6% (95% CI 44-71) and 37.9% (95% CI 25-51). According to donor type, 5-yr OS was 69%, 39% and 89% and DFS 57%, 19% and 57% for HLA-identical sibling, UD and Haplo, respectively (Figure 1). 5-yr OS was significantly higher in SS versus MF patients (78% vs 47%, p=0.03) and in patients who were in CR at the time of transplant (100% vs 43.5%, p<0.001).
In the Haplo setting,.disease status at transplant was CR in 1 pt and PR in 8. After a median follow-up of 26 months, 7 out of 9 patients (78%) were still alive and in CR. Acute GvHD occurred in 6 patients (66%), grade III-IV in 2 (22%), while chronic GvHD occurred in 3 patients (33%), moderate/severe in 1 (11%). Two patients relapsed at +5 and +2 months, of whom the latter died 157 days after HCT.
Conclusions: After a median follow up of more than 9 years for alive patients, we confirm that RIC allo-HSCT is a powerful therapeutic strategy in selected patients with advanced CTCL, and particularly in SS. With the limitation of a shorter follow-up, transplant outcomes following allo-HCT from haploidentical donor appear to be associated with very low toxicity, excellent OS and DFS comparable to HLA-identical sibling transplants.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal